A Major Review of Optical Coherence Tomography Angiography
- Review
- Open Access
- Published:
A review of optical coherence tomography angiography (OCTA)
International Journal of Retina and Vitreous volume ane, Article number:5 (2015) Cite this article
Abstract
Optical coherence tomography angiography (OCTA) is a new, non-invasive imaging technique that generates volumetric angiography images in a matter of seconds. This is a nascent technology with a potential wide applicability for retinal vascular disease. At nowadays, level i evidence of the technology'southward clinical applications doesn't exist. In this paper, nosotros introduce the engineering science, review the available English linguistic communication publications regarding OCTA, and compare information technology with the current angiographic gold standards, fluorescein angiography (FA) and indocyanine green angiography (ICGA). Finally we summarize its potential application to retinal vascular diseases. OCTA is quick and non-invasive, and provides volumetric data with the clinical capability of specifically localizing and delineating pathology along with the power to show both structural and blood flow data in tandem. Its current limitations include a relatively modest field of view, inability to prove leakage, and proclivity for paradigm antiquity due to patient movement/blinking. Published studies hint at OCTA'south potential efficacy in the evaluation of common ophthalmologic diseases such age related macular degeneration (AMD), diabetic retinopathy, avenue and vein occlusions, and glaucoma. OCTA tin discover changes in choroidal blood vessel menstruation and tin elucidate the presence of choroidal neovascularization (CNV) in a diversity of weather but specially in AMD. It provides a highly detailed view of the retinal vasculature, which allows for authentic delineation of the foveal avascular zone (FAZ) in diabetic eyes and detection of subtle microvascular abnormalities in diabetic and vascular occlusive eyes. Optic disc perfusion in glaucomatous eyes is notable every bit well on OCTA. Further studies are needed to more definitively determine OCTA's utility in the clinical setting and to establish if this technology may offer a non-invasive option of visualizing the retinal vasculature in particular.
Introduction
Optical coherence tomography angiography (OCTA) is a new non-invasive imaging technique that employs motility contrast imaging to high-resolution volumetric blood flow information generating angiographic images in a matter of seconds. OCTA compares the decorrelation bespeak (differences in the backscattered OCT signal intensity or aamplitude) between sequential October b-scans taken at precisely the same cross-section in gild to construct a map of blood flow. Axial bulk motility from patient motion is eliminated and so sites of motion between repeated OCT b-scans represent strictly erythrocyte movement in retinal blood vessels [1-4].
OCTA requires higher imaging speeds than most currently available OCT systems can provide in order to obtain a densely sampled volume. Conventional October device scanning speeds would effect in as well much trade-off between decreased field of view, lower image quality, and greatly increased scanning time.
Comparison OCTA with FA and ICGA
Fluorescein angiography (FA) and indocyanine green angiography (ICGA) are both invasive test that require intravenous assistants of dye and imaging up to 10–30 minutes [v-9]. They provide ii-dimensional image sets that permit for dynamic visualization of claret flow with a wide field of view. Therefore, patterns of dye leakage, pooling, and staining can exist appreciated and are well-documented in the literature [10]. FA remains the golden standard for the detection of choroidal neovascularization (CNV), also as retinal neovascularization such every bit neovascularization of the disc (NVD) and neovascularization elsewhere (NVE) [11-thirteen]. However, retinal pathology can exist obscured by this leakage also as hemorrhage or media opacities, and localization of the depth of the lesion and size delineation of neovascularization can be hard due to dye leakage and poor stereopsis, and because the imaging modalities are not depth resolved. As a result, segmentation of different layers is not routinely possible with FA or ICGA. Therefore, identification of the axial location of pathology requires an understanding of patterns of blockage and leakage [10]. For example, differentiation betwixt blazon ane CNV, which is found between the retinal paint epithelium (RPE) and Bruch'due south membrane, and type 2 CNV, which is constitute in the subretinal infinite above the RPE, requires understanding that the RPE blocks underlying fluorescence so type 1 CNV requires a larger amount of dye to accumulate before hyperfluorescence is credible [xiv].
FA and ICGA have other drawbacks that tin can limit their widespread use. Since they are invasive, relatively expensive, and time-consuming, they are non ideal techniques to use on a regular footing in a busy clinical setting. Although considered safe, the dyes pose risks ranging from nausea to allergic reactions, including anaphylaxis in rare instances. Aside from allergic reactions of which the likelihood increases with frequency of use, indocyanine green dye is contraindicated in pregnancy and kidney affliction [15-17]. For the evaluation of patients requiring frequent follow-up exams or of those that may not tolerate injection of intravenous dye, a rapid non-invasive technique to visualize retinal and choroidal vessels would be beneficial.
OCTA in comparing is a non-invasive technique that acquires volumetric angiographic information without the use of dye. Each 3-dimensional scan set takes approximately six seconds to obtain. The en-face images (OCT angiograms) can so be scrolled outward from the internal limiting membrane (ILM) to the choroid to visualize the individual vascular plexus and segment the inner retina, outer retina, choriocapillaris, or other area of involvement. The en-face acquisition areas currently range from 2 × two mm to 12 × 12 mm with the scan quality greatly decreased with a widened field of view since the same number of OCT b-scans is used for all scanning areas. The 12 x 12 mm browse is only bachelor on inquiry prototypes. The 3 × three mm Oct angiograms appear to be higher resolution than the currently available FA/ICGA images, and a study by Matsunaga et al. deduced that they were at least equivalent in showing important vascular particular [18]. Utilize of the montage technique allows for a larger field of view much like FA/ICGA while maintaining this improved resolution (Effigy 1; de Carlo TE et al., unpublished information in review). Carl Zeiss, Inc (Carl Zeiss Meditec, Dublin, CA) is developing an automated broad-field montage software, which employs movement tracking to rail the optics and stitch images together.
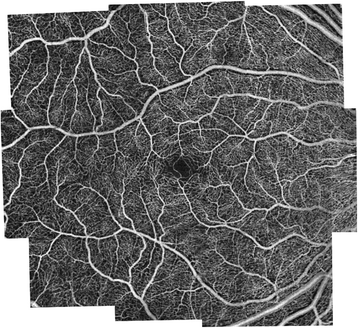
OCTA Wide-Field Montage of a Normal Eye. Optical coherence tomography angiography (OCTA) broad-field montage of the normal right centre of a 56 year old Caucasian human. Images were acquired using the Angiovue software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA) and montaged using Adobe Photoshop (San Jose, CA). This allows for a larger field of view much similar fluorescein and indocyanine dark-green angiography while maintaining improved resolution (de Carlo TE et al., unpublished data in review).
OCTA provides menstruum information at a stock-still point in time. Although leakage is not appreciable, exact delineation and size measurements tin be performed for pathology such as CNV (de Carlo TE et al., unpublished data in review) [19]. This is particularly useful for identification of blazon ane CNV where localization is inferential and therefore may exist inaccurate with FA/ICGA. Retinal blood flow on OCTA tin exist obscured by hemorrhage as this decreases the ability of low-cal to penetrate into the deeper layers of the eye.
OCTA provides both structural and functional (i.e. claret catamenia) information in tandem. The "respective" OCT b-scans can be co-registered with the simultaneous Oct angiograms so the operator is able to scroll through the OCT angiogram like a cube browse. As a result, the precise location of pathology can be viewed on the corresponding OCT b-scans. The axial resolution of the corresponding Oct b-scans are lower quality than the typical highly-sampled line scans and are similar to the resolution of individual Oct b-scans inside a volumetric cube scan. Both the retinal and the choroidal microvasculature can be visualized using OCTA while FA is used for seeing the retinal vessels and ICGA is more platonic for imaging the choroid.
Using the present technology, OCTA is more than decumbent to artifact than FA or ICGA. The larger retinal vessels cause a "ghost image" referred to as a shadow antiquity, when segmenting deeper layers, particularly the outer retina. This can brand it more difficult to appreciate the presence of abnormal vasculature in the deeper layers. Because OCTA uses the principle that movement in the back of the eye represents blood menstruation, it is prone to move artifact. White lines (representing decorrelation signal over the entire b-scan) announced in areas of bulk patient move such as when the patient loses fixation or moves. Conversely, blinks appear as a blackness line beyond the OCT angiogram because the Oct signal is blocked from reaching the retina and the software, therefore, detects no movement. Although erythrocytes should exist the simply moving object in the retina, some non-vascular structures such as fine tissue may also cause a decorrelation signal, especially if the patient is moving. For instance, the edges of a retinal pigment epithelial detachment (RPED) often testify up on OCTA as white dissonance antiquity in cases of increased patient motion. Information technology is postulated that because the RPE is a fine structure, in areas of disruption such as a RPED, information technology can presumably move and therefore be detected on the OCT angiogram.
On the other hand, OCTA can besides miss areas of dull blood flow such as in microaneurysms or fibrotic CNV. Since OCTA relies on change betwixt consecutive b-scans, it will notice period only in a higher place a minimum threshold, the slowest detectable menstruum, which is determined by the time between the ii sequential Oct b-scans. Lesions that accept flow beneath the slowest detectable flow would therefore not be visualized using this imaging technique. Increasing the fourth dimension between consecutive Oct b-scans could let for increased flow detection but would offer a merchandise-off due to increased motion artifact. One of the advantages of a higher speed organisation is that multiple volumetric sets tin exist obtained at each cantankerous-section and so the threshold tin be altered later by selecting dissimilar fourth dimension frames between the October b-scans to decide the optimal prototype quality. Therefore if a low-catamenia vessel is undetectable by using the first and 2d Oct b-scans at a given cantankerous section, the image may be processed using the first and 3rd Oct b-scans to increment the time betwixt the Oct b-scans thereby decreasing the minimum threshold.
A couple of publications have qualitatively compared OCTA with FA. Spaide et al. described the peripapillary retinal vascular layers in 12 normal eyes, finding that OCTA provided improved visualization of all the vascular layers including the radial peripapillary and deep capillary networks that were non well-distinguished on FA [3]. OCTA imaging of the perifoveal region was reported by Matsunaga et al., demonstrating that the ability to see the normal retinal vasculature was equivalent to that of FA [18].
Review
OCTA of normal optics
The most widely available image OCTA system is the AngioVue software of the RTVue XR Avanti spectral-domain Oct (SD-October) (Optovue, Inc, Fremont, CA), which uses a split up-spectrum aamplitude decorrelation angiography (SSADA) algorithm. The device obtains volumetric scans of 304 × 304 A-scans at seventy,000 A-scans per second in approximately iii.0 seconds. The software offers the selection of two × 2 mm, 3 × 3 mm, 6 × 6 mm, and viii × viii mm October angiograms (Figure 2A-C) and automatic segmentation of these full-thickness retinal scans into the "superficial" and "deep" inner retinal vascular plexuses, outer retina, and choriocapillaris (Figure 2E-H). The OCT angiogram segmentation of the superficial inner retina contains a projection of the vasculature in the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) (Effigy 2E). The deep inner retina OCT angiogram partition shows a composite of the vascular plexuses at the border of the inner plexiform layer (IPL) and inner nuclear layer (INL) and the border of the INL and outer plexiform layer (OPL) (Figure 2F).
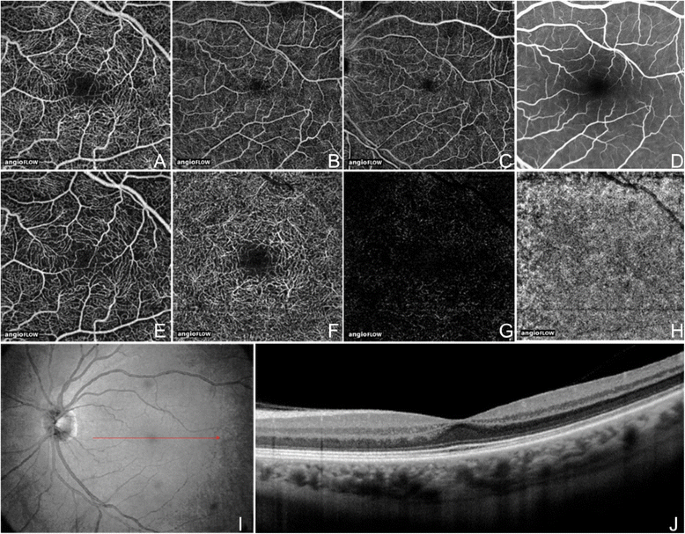
OCT Angiogram Fields of View and Partition Layers on Angiovue. The normal left heart of a 56 year old Caucasian man using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A) Full-thickness (internal limiting membrane to Bruch's membrane) three x iii mm OCT angiogram. (B) Total-thickness 6 ten 6 mm OCT Angiogram. (C) Full-thickness eight x 8 mm October Angiogram. (D) Fluorescein angiography cropped to approximately 8 x 8 mm or thirty degrees demonstrates less capillary detail than A-C. (E) 3 10 3 mm Oct angiogram of the "Superficial" inner retina. (F) 3 ten three mm OCT angiogram of the "Deep" inner retina. (G) 3 x 3 mm OCT angiogram of the outer retina shows absence of vasculature. The white represents noise. (H) three x 3 mm OCT angiogram of the choriocapillaris is generally homogenous. There is black shadowing from retinal vessels. (I) En-face up intensity OCT image. (J) Highly-sampled October b-scan image.
The OCTA prototype with the fastest acquisition rate was developed by the Massachusetts Institute of Technology using a swept-source OCT (SS-Oct) device (Department of Electrical Engineering and Computer science and Inquiry Laboratory of Electronics, Massachussetts Institute of Engineering science, Cambridge, MA). This ultra-high speed prototype employs a vertical cavity surface emitting laser (VCSEL) operating at 1060 nm wavelength which allows increased low-cal penetration into pigmented tissues and improved choroidal claret flow visualization compared to the lite source used in SD-Oct. The SS-OCTA system obtains scans of 500 × 500 A-scans at 400,000 A-scans per second in approximately 3.eight seconds. This ultra-high speed allows for imaging of wider fields of view. The prototype can be manipulated to obtain OCT angiograms up to 12 × 12 mm, nevertheless, it is most usually used to create 3 × iii mm and half-dozen × 6 mm Oct angiograms of dandy detail (Figure 3A-B). Full-thickness scans are manually segmented into the superficial (plexus at the RNFL), intermediate (plexus at the GCL), and deep (plexuses at IPL/INL and INL/OPL borders) inner retinal vascular plexuses, outer retina, choriocapillaris, and choroidal layers (Effigy 3D-F). Using this OCTA system, the choriocapillaris and choroidal vessels were described in normal eyes by Choi et al [ii].

OCT Angiogram Fields of View and Segmentation Layers on the SS-October Protype. The normal correct eye of a 26 yr old Caucasian woman using a image swept source optical coherence tomography angiography (OCTA) system (Department of Electrical Applied science and Information science and Research Laboratory of Electronics, Massachussetts Insitute of Technology, Cambridge, MA). (A) Full-thickness (internal limiting membrane to Bruch'southward membrane) 3 x 3 mm October angiogram. (B) Full-thickness 6 x half dozen mm OCT angiogram. (C) Respective October b-browse. (D) 3 ten 3 mm October angiogram of the retinal nervus cobweb layer plexus of the inner retina. (Due east) 3 x 3 mm OCT angiogram of the ganglion jail cell layer plexus of the inner retina. (F) iii x 3 mm Oct angiogram of the "deep" inner retina.
OCTA of dry (Not-Neovascular) AMD
Dry age-related macular degeneration (AMD) is characterized by drusen, pigmentary changes, and photoreceptor and RPE loss, called geographic cloudburst (GA). Decreased foveolar choroidal claret menstruum is associated with AMD and increased drusen extent, and it has been hypothesized that the choroidal blood menses may predict disease progression [14]. Choi et al. (unpublished data, presented in part at the Association for Enquiry in Vision and Ophthalmology annual coming together, May 2014, Orlando, Florida) demonstrated OCTA findings in dry out AMD. Areas of impaired choriocapillaris menstruation typically extended beyond the borders of the GA. Eyes with dry out AMD were shown to have a generalized subtract in choriocapillaris density, which was sometimes associated with drusen. Figures 4 and v demonstrate discrete areas of decreased point at the choriocapillaris level below many but not all drusen in 3 eyes. These areas of alteration did non appear to be due to shadowing (from material in the drusen), and some choroidal vessels were appreciated below these areas. However, further studies would be necessary to determine if the choriocapillaris changes associated with the drusen are true areas of catamenia damage. Choriocapillaris flow alterations are besides shown in two eyes along the border of GA in Figure half-dozen.
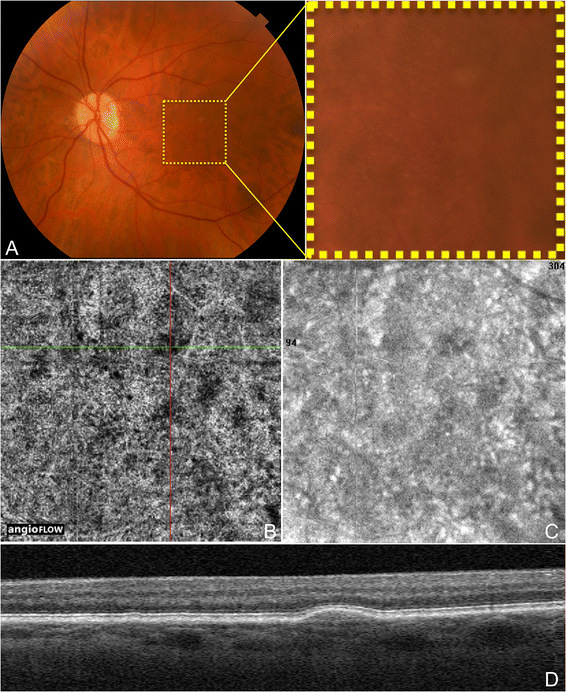
OCTA and Colour Fundus Photograph of Drusen in Non-Neovascular AMD. The left eye of a 72 twelvemonth old Caucasian human being with non-neovascular age-related macular degeneration using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A) Colour fundus photo zoomed in to an approximately three ten 3 mm area centered at the macula showing hard and soft drusen. (B) 3 x iii mm OCT angiogram of the choriocapillaris centered at the macula every bit in A. The green and ruby-red lines represent the x and y axis OCT b-scans respectively which cantankerous at a soft druse demonstrating an area of decreased indicate in the choriocapillaris underlying the druse. (C) 3 x 3 mm en-face structural October of the choriocapillaris centered at the macula equally in A-B. This image was simultaneously obtained during the aforementioned browse as the October angiogram in B. This structural October is still able to show the choriocapillaris changes at the location of the soft drusen in B, but detail is overall limited. (D) Corresponding x axis October b-scan at the cross-department demonstrated by the light-green line in B showing the soft druse. The corresponding OCT b-scans were simultaneously obtained during the same browse as the OCT angiogram in B.
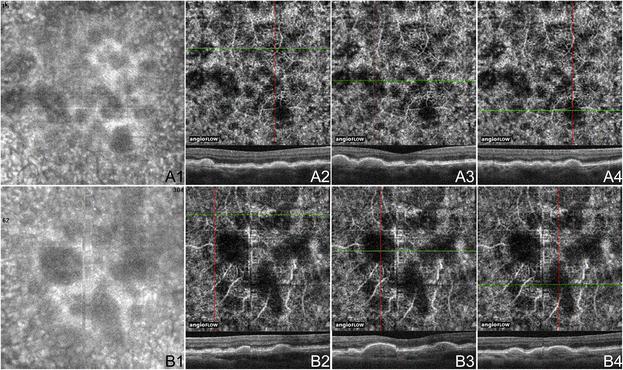
OCTA of Drusen in Non-Neovascular AMD Cases. (A) iii x three mm en-face images of the right eye of a 74 yr old Caucasian human with non-neovascular age-related macular degeneration (AMD) using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) En-confront structural Oct demonstrating areas of choriocapillaris alteration. (A2-four) Oct angiograms of the choriocapillaris and corresponding x-axis October b-scans at the cross-sections shown past the greenish line of the October angiograms. The 3 soft drusen shown are associated with areas of decreased bespeak in the choriocapillaris, which could betoken flow impairment. (B) 3 x 3 mm en-confront images of the left centre of an 80 year old Asian woman with not-neovascular AMD using the Angiovue OCTA software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (B1) En-face structural OCT demonstrating areas of choriocapillaris changes. (B2-iv) October angiograms of the choriocapillaris and corresponding x-axis October b-scans at the cross-sections shown by the greenish line of the Oct angiograms. The druse in B2 is not associated with choriocapillaris loss. The other 2 soft drusen shown represent to areas of decreased bespeak in the choriocapillaris, which could indicate catamenia impairment.
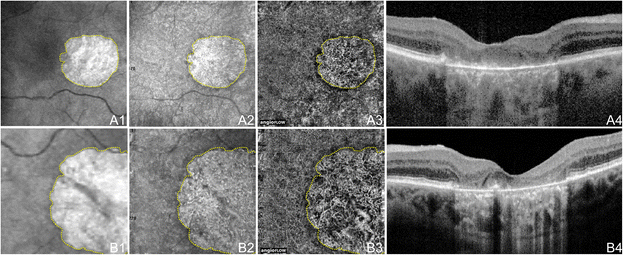
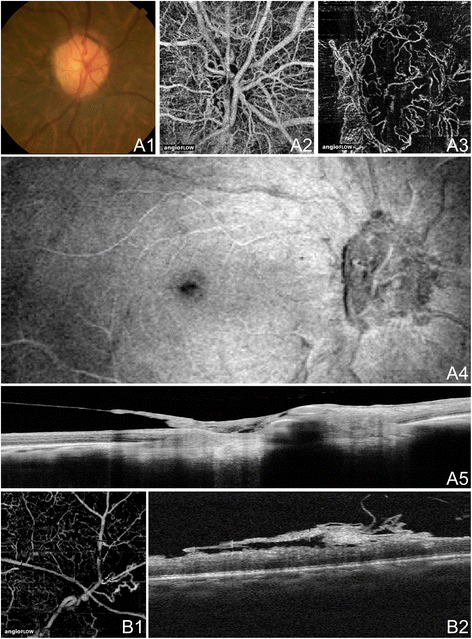
OCTA of GA in Non-Neovascular AMD. 71 year old Caucasian man with geographic atrophy (GA) due to not-neovascular age-related macular degeneration using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A) 6 x 6 mm en-confront images of the correct eye. (A1) En-face structural OCT at the level of the RPE demonstrating GA. The area of GA is circumscribed in yellow, which is overlaid over the images in A2 and A3. (A2) En-face structural Oct at the level of the choriocapillaris demonstrating alteration in a like area equally the GA. (A3) Oct angiogram at the level of the choriocapillaris demonstrating flow impairment in a similar expanse every bit the GA. Larger choroidal vessels have been button inwards into the area of choriocapillaris alteration so are seen in this 10micrometer piece. Detail is greatly improved over that of the en-face structural OCT. (A4) Corresponding Oct b-scan shows the loss of RPE causing increased intensity below Bruch'southward membrane which is characteristic of GA. (B) 3 x iii mm en-face images of the left eye. (B1) En-face up structural OCT at the level of the RPE demonstrating GA. The area of GA is confining in yellow, which is overlaid over the images in B2 and B3. (B2) En-face structural OCT at the level of the choriocapillaris demonstrating amending in a similar area equally the GA. (B3) October angiogram at the level of the choriocapillaris demonstrating flow impairment in a similar expanse equally the GA. Larger choroidal vessels have been push inward into the area of choriocapillaris alteration and so are seen in this 10micrometer slice. Detail is greatly improved over that of the en-face structural OCT. (B4) Respective OCT b-scan shows the loss of RPE causing increased intensity below Bruch'southward membrane which is feature of GA.
OCTA of moisture (Neovascular) AMD
Several publications concerning OCTA of eyes with moisture AMD appear in the literature. In July 2014 Jia et al. get-go described the power of a prototype SS-OCTA system to visualize and quantify CNV that had been seen on FA in five eyes [19]. And so in November 2014, Moult and Choi et al. described CNV in 16 of 19 eyes with neovascularization, noting that the majority of these eyes (14/16, 88%) also demonstrated choriocapillaris alteration surrounding the CNV [20]. De Carlo et al. described qualitative and quantitative characteristics of CNV in 48 eyes [21]. The group determined sensitivity and specificity of the image AngioVue software, using FA as the ground truth, to be 50% (four/8) and 91% (twenty/22) respectively, hypothesizing that the low sensitivity was due to small sample size and blockage from big amounts of retinal hemorrhage in some patients. Figures vii and eight illustrate three examples of CNV, including i blazon 3 CNV (retinal angiomatous proliferation, RAP), on OCTA confirmed with FA/ICGA, using the Angiovue OCTA software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). Figure nine shows 2 OCTA examples of CNV, 1 of which was treatment naïve, using the SS-Oct prototype (Department of Electric Engineering and Informatics and Inquiry Laboratory of Electronics, Massachussetts Insitute of Technology, Cambridge, MA).

OCTA and FA/ICGA of CNV in Neovascular AMD. The left eye of a 67 year old Caucasian man with choroidal neovascularization (CNV) due to neovascular age-related macular degeneration using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A) half dozen x 6 mm October angiogram segmented so both the choriocapillaris and the outer retina are shown. A circular net of abnormal vessels are shown surrounded past relatively homogenous choriocapillaris. The abnormal vessels be both beneath and above Bruch's membrane (in the outer retina). (B) En-face structural OCT with a ruby line corresponding to the highly-sampled OCT b-scan in C. (C) 12 mm highly sampled OCT b-scan through the fovea demonstrates a large retinal pigment epithelial disengagement, subretinal fluid, disruption of Bruch'south membrane, and hyper-cogitating material characteristic of CNV. (D) Indocyanine dark-green angiography early, intermediate, and late frames show increasing hyper-fluorescence and pooling of dye in the CNV. (E) Fluorescein angiography intermediate and late frames show increasing hyper-fluorescence and pooling of the CNV.
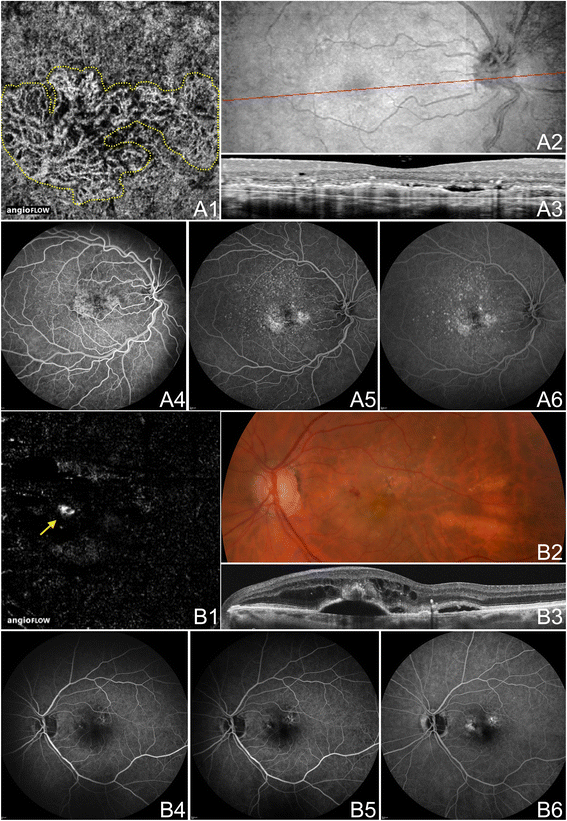
OCTA and FA of CNV in Neovascular AMD. (A) The right middle of a 63 year onetime Caucasian human with choroidal neovascularization (CNV) due to neovascular age-related macular degeneration (AMD) using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) 3 10 three mm OCT angiogram segmented then both the choriocapillaris and the outer retina are shown. Ii nets of abnormal vessels are shown surrounded past relatively homogenous choriocapillaris. The abnormal vessels exist both beneath and above Bruch's membrane (in the outer retina). (A2-3) En-confront structural Oct with a cherry line corresponding to a 12 mm highly sampled October b-scan (cropped to three mm) through the macula. The OCT b-scan demonstrates a retinal pigment epithelial detachment (RPED), subretinal fluid, an intraretinal cyst, and hyper-reflective material characteristic of CNV. (A4-6) Fluorescein angiography (FA) early on, intermediate, and late frames showing increasing hyper-fluorescence and staining of the CNV. (B) The left middle of an 89 year quondam Caucasian adult female with CNV type three (retinal angiomatous proliferation, RAP) due to neovascular AMD using the Angiovue OCTA software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (B1) half-dozen x 6 mm October angiogram segmented at the outer retina showing a round RAP lesion (yellowish pointer). A feeder vessel from a retinal vessel was noted (not shown). (B2) Color fundus photograph demonstrating hemorrhage in the region of the RAP lesion. (B3) vi mm highly sampled Oct b-scan through the macula shows subretinal and intraretinal fluid and a round brawl of hyper-reflective tissue above a serous RPED. (B4-6) FA early, intermediate, and late frames showing increasing hyper-fluorescence and pooling in the CNV.
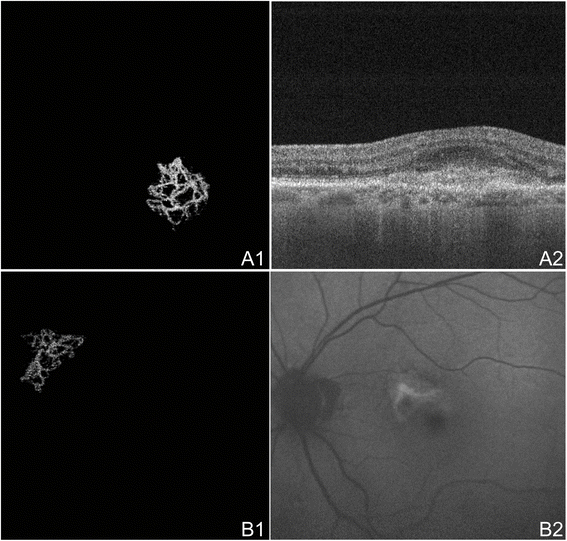
OCTA of CNV in Neovascular AMD. (A) The left eye of an 89 year old Caucasian human with choroidal neovascularization (CNV) due to neovascular age-related macular degeneration (AMD) using the swept source optical coherence tomography angiography (OCTA) prototype (Department of Electrical Applied science and Reckoner Science and Research Laboratory of Electronics, Massachussetts Insitute of Engineering, Cambridge, MA). (A1) 3 ten 3 mm OCT angiogram of the outer retina with transmission removal of the retinal vessel ghost artifact. A body of water-fan appearing CNV is seen. (A2) Corresponding October b-scan showing a retinal pigment epithelial detachment, disruption of Bruch's membrane, and hyper-reflective textile feature of CNV. (B) The left centre of a 70 year quondam Caucasian man with treatment-naïve choroidal neovascularization (CNV) due to neovascular age-related macular degeneration (AMD) using the swept source OCTA paradigm (Department of Electrical Applied science and Figurer Science and Enquiry Laboratory of Electronics, Massachussetts Insitute of Technology, Cambridge, MA). (B1) 3 x 3 mm October angiogram of the outer retina with transmission removal of the retinal vessel ghosting artifact. A sea-fan appearing CNV is seen. (B2) Ruddy-free fundus photo exhibiting a lesion of the same shape and location as the CNV seen in B1.
OCTA of diabetes
There are few published papers equally of early on 2015 on OCTA of diabetic retinopathy. Choi et al. (unpublished data) demonstrated that OCTA of diabetic eyes ranging from no retinopathy to proliferative diabetic retinopathy (PDR) demonstrated choriocapillaris abnormalities and/or retinal microvascular abnormalities such every bit microaneurysms, vascular remodeling adjacent to the foveal avascular zone (FAZ), enlarged FAZ, and capillary tortuosity and dilation. OCTA and FA were compared in unpublished data past Salz et al. The group supported the utility of OCTA in evaluating FAZ and the perifoveal intercapillary surface area, showing that they were sequentially enlarged in each stages of diabetic retinopathy (normal eyes to PDR). The data showed that OCTA visualized the majority merely not all of the microaneurysms visualized past FA probable because OCTA is limited by the principle of slowest detectable flow. Nonetheless, OCTA was able to appreciate some microaneurysms that were non detected past FA. OCTA as well successfully detected other abnormalities that were not evident on FA such as areas of retinal non-perfusion, reduced capillary density, and increased vessel tortuosity. de Carlo et al. (unpublished data in review) described a wide-field OCTA montage of an centre with newly proliferative diabetic retinopathy. The wide-field montage OCTA prototype also successfully allowed visualization of an enlarged FAZ, perifoveal intercapillary area, and multiple microaneurysms. It also provided a larger field of view allowing more peripheral detection of microvascular changes, early NVE, and areas of capillary not-perfusion including areas too small to visualize on FA.
Figure ten shows an enlarged FAZ on OCTA and compares OCTA and FA in the identification of microaneurysms in two eyes with not-proliferative diabetic retinopathy (NPDR). Capillary non-perfusion and other retinal microvascular abnormalities are demonstrated in Effigy 11. OCTA examples of NVD and NVE in PDR eyes are shown in Effigy 12.
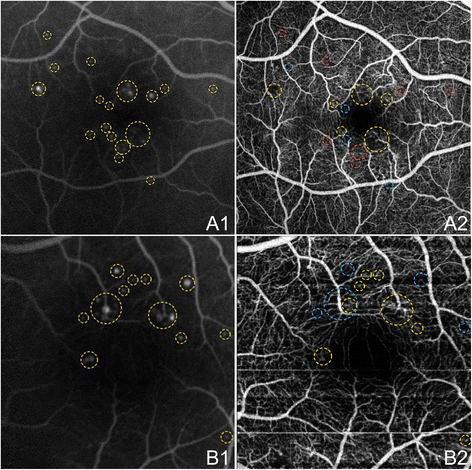
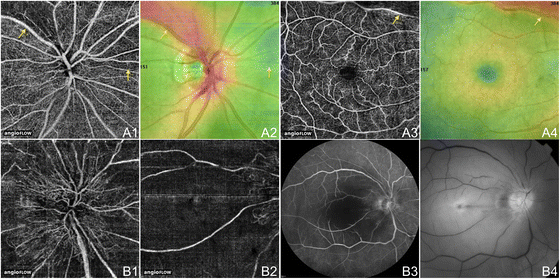
OCTA and FA of Microaneurysms in NPDR. The right centre (A) and left centre (B) of a 45 year onetime Caucasian human being with non-proliferative diabetic retinopathy using the swept source optical coherence tomography angiography (OCTA) prototype (Section of Electric Engineering and Computer Scientific discipline and Research Laboratory of Electronics, Massachussetts Insitute of Applied science, Cambridge, MA). (A1) Fluorescein angiography (FA) cropped to approximately 6 10 6 mm. Aneurysms are circled in xanthous. (A2) Full-thickness (internal limiting membrane to Bruch'due south membrane) 6 ten 6 mm OCT angiogram. FAZ appears enlarged. Aneurysms that are seen on FA in A1 that are also seen on OCTA are circled in yellow. Aneurysms on FA that are seen as areas of capillary non-perfusion on OCTA are circled in blue. Areas where aneurysms are seen on FA, simply evidence normal vasculature on OCTA are circled in scarlet. (B1) FA cropped to approximately 3 x 3 mm. Aneurysms are circled in yellow. (B2) Full-thickness iii ten 3 mm October angiogram, which provides improved detail over 6 10 half-dozen mm OCT angiograms, demonstrates higher sensitivity in detecting micro vascular abnormalities. FAZ appears enlarged. Aneurysms that are seen on FA in B1 that are as well seen on OCTA are circled in yellow. Aneurysms on FA that are seen equally areas of capillary not-perfusion on OCTA are circled in blue.
OCTA of NPDR. The correct middle (A) and left eye (B) of a 58 twelvemonth onetime Caucasian man with not-proliferative diabetic retinopathy and diabetic macular edema (DME) using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) Full-thickness (internal limiting membrane to Bruch's membrane) 6 x 6 mm OCT angiogram shows microvascular abnormalities such as areas of capillary non-perfusion (yellow arrows), capillary loops, and microaneurysms. (A2) En-face structural Oct with a red line corresponding to the highly-sampled OCT b-scan in A3. (A3) 12 mm highly sampled October b-scan through the fovea demonstrating DME and hard exudates. (B1) Full-thickness three 10 3 mm OCT angiogram, which provides improved detail over 6 x 6 mm OCT angiograms, shows microvascular abnormalities such as areas of capillary non-perfusion (yellow arrows), capillary loops, and microaneurysms. (B2) En-face structural October with a cherry line respective to the highly-sampled OCT b-scan in B3. (B3) 12 mm highly sampled Oct b-scan through the fovea demonstrating DME and hard exudates.
OCTA of Neovascularization in PDR. (A) The right eye of a 74 year old African woman with neovascularization of the disc (NVD) due to proliferative diabetic retinopathy (PDR) using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) Color fundus photo demonstrating fine neovascular vessels over the optic disc. (A2) Full-thickness (internal limiting membrane to Bruch'south membrane) iii 10 3 mm OCT angiogram at the optic disc. Wispy NVD is difficult to appreciate. (A3) 3 10 3 mm October angiogram at the optic disc segmented with the inner boundary in the vitreous above the NVD and the outer boundary slightly below the internal limiting membrane (ILM). The NVD is clearly appreciable. (A4) En-face structural Oct showing abnormal tissue at the optic disc. (A5) Highly-sampled OCT b-scan of the optic disc where abnormal tissue is observed extending above the ILM into the vitreous crenel. (B) The right eye of a 46 year old African woman with neovascularization elsewhere (NVE) due to proliferative diabetic retinopathy (PDR) using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (B1) 3 x three mm OCT angiogram with the inner boundary in the vitreous and the outer boundary at Bruch's membrane. Abnormal vessels are seen in an expanse of capillary non-perfusion. Paradigm quality is limited past antiquity from movement (horizontal and vertical lines). (B2) Corresponding October b-scan showing aberrant tissue above the ILM extending into the vitreous cavity.
OCTA of artery and vein occlusion
Retinal vascular occlusions have yet to be described in the literature using OCTA as an imaging modality. However, preliminary work at the New England Eye Center of Boston, MA shows that OCTA may be useful for evaluating these diseases. Unpublished data in review by de Carlo et al. described a case of co-operative retinal vein apoplexy (BRVO) using a wide-field montage technique. The OCTA showed a big wedge-shaped area of capillary non-perfusion in the inferotemporal macula with articulate delineation of the purlieus of ischemia, and vascular abnormalities such equally microaneurysms, telangiectasis, and anastamoses.
Figure thirteen shows OCT angiograms of an astute branch retinal avenue occlusion (BRAO) and a subacute primal retinal artery occlusion (CRAO). The BRAO demonstrates wedge-shaped areas of capillary non-perfusion that correlate to areas of abnormalities on the retinal thickness map. This illustrates the potential use of OCTA in pinpointing areas of ischemia and edema. The CRAO shows diffuse capillary non-perfusion in areas supplied by the central retinal avenue as seen on the same-day FA. Menses is withal seen in the major retinal vessels. Around the optic disc, there is an absence of blood menstruum in the superficial disc vasculature supplied past the central retinal artery just the lamina cribosa claret flow remains intact. Every bit OCTA provides a snapshot in fourth dimension, information technology does not demonstrate delayed arteriovenous transit fourth dimension as FA does.
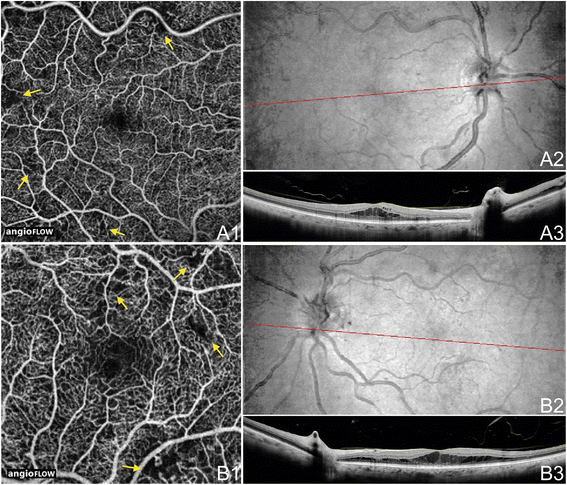
OCTA of BRAO and CRAO. (A) The correct eye of a 70 year old Caucasian man with an astute branch retinal artery occlusion using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) Full-thickness (internal limiting membrane to Bruch's membrane) 4.5 ten 4.5 mm Oct angiogram of the optic disc showing decreased capillary perfusion superotemporal and nasal to the disc (yellowish arrows). (A2) four.5 x 4.5 mm en-face OCT thickness map showing retinal thickening in cerise and thinning in blue (yellow arrows) that correspond to the decreased capillary perfusion in A1. (A3) Total-thickness 6 ten 6 mm OCT angiogram illustrating decreased capillary perfusion superotemporal and nasal to the disc (xanthous pointer) as in A1. (A4) half-dozen 10 6 mm en-face October thickness map showing retinal thickening in cherry (xanthous arrow) that represent to the decreased capillary perfusion in A3. (B) The right eye of an 81 year former Caucasian man with a subacute central retinal artery apoplexy using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (B1) Full-thickness iv.five 10 four.5 mm OCT angiogram of the optic disc showing diffusely decreased peripapillary capillary perfusion. (B2) Full-thickness 6 10 half-dozen mm OCT angiogram illustrating decreased capillary perfusion in the macula. Only the large retinal and peripapillary vessels demonstrate blood flow. (B3) Fluorescein angiography is hypo-fluorescent in the macula and peripapillary region due to the decreased ability for the fluorescein dye to attain these areas because of low blood flow. The vessels appear adulterate. (B4) Ruby-free fundus photograph demonstrates attenuation of the vessels especially in the macular and peripapillary regions.
A instance of BRVO and a case of central retinal vein occlusion (CRVO) are illustrated in Figure xiv. OCTA of the BRVO shows capillary not-perfusion superotemporally forth the superior arcade extending into the FAZ, and telangiectatic vessels, capillary loops, and possible microaneurysms at the edge of the ischemic areas. The OCTA of the chronic CRVO demonstrates diffuse capillary not-perfusion continuous with the FAZ and telangiectatic vessels.
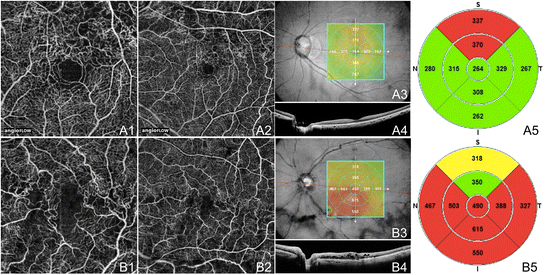
OCTA of BRVO and CRVO. (A) The left center of a 61 year old Asian woman with a chronic branch retinal vein apoplexy using the Angiovue optical coherence tomography angiography (OCTA) software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (A1) Total-thickness (internal limiting membrane to Bruch's membrane) three x iii mm OCT angiogram showing capillary not-perfusion superotemporal extending into the foveal avascular zone (FAZ) and telangiectatic vessels at the border of the ischemic areas. (A2) Full-thickness 6 x six mm October angiogram demonstrating that the capillary not-perfusion is along the superior arcade. The edges of the ischemia are bordered by telangiectatic vessels, capillary loops, and possible microaneurysms. (A3) En-face structural OCT with a retinal thickness map and a ruby line corresponding to the highly-sampled OCT b-scan in A4. (A4) 12 mm highly sampled OCT b-scan through the fovea which appears relatively unaffected. (A5) Retinal thickness map demonstrating superior thickening due to edema. (B) The left eye of a 72 year old Caucasian homo with a chronic fundamental retinal vein apoplexy using the Angiovue OCTA software of the RTVue XR Avanti (Optovue, Inc., Fremont, CA). (B1) Full-thickness iii 10 3 mm October angiogram showing lengthened capillary non-perfusion continuous with the FAZ and telangiectatic vessels. (B2) Total-thickness 6 x 6 mm OCT angiogram demonstrating telangiectatic vessels and diffuse capillary non-perfusion especially along the junior arcade. (B3) En-face up structural OCT with a retinal thickness map and a red line corresponding to the highly-sampled OCT b-browse in B4. (B4) 12 mm highly sampled OCT b-browse through the fovea which shows macular edema and disruption of the photoreceptor layer. (B5) Retinal thickness map demonstrating thickening that is greatest inferiorly.
OCTA of glaucoma
OCTA is a useful tool for evaluating optic disc perfusion in glaucomatous eyes. The normally dense peripapillary microvascular network is attenuated in both the superficial disc vasculature and the deeper lamina cribosa. Averaging the decorrelation signal in Oct angiograms approximates the expanse of microvasculature and allows the user to calculate the flow index, which is decreased in eyes with glaucoma. The flow index has been shown to have both a very high sensitivity and specificity in differentiating glaucomatous eyes from normal eyes [22,23].
Conclusions
OCTA is a new technology that has great potential for utilize in the clinical setting. Compared with FA and ICGA, the current retinal angiographic gold standards, OCTA advantages are that information technology is non-invasive, acquires volumetric scans that can be segmented to specific depths, uses motion contrast instead of intravenous dye, tin can exist obtained within seconds, provides authentic size and localization information, visualizes both the retinal and choroidal vasculature, and shows structural and blood flow information in tandem. Disadvantages of OCTA are its limited field of view, inability to view leakage, increased potential for artifacts (blinks, movement, vessel ghosting), and disability to notice blood period below the slowest detectable flow.
OCTA has been shown to exist a useful imaging modality for the evaluation of mutual ophthalmologic diseases such AMD, diabetic retinopathy, artery and vein occlusions, and glaucoma. In some cases OCTA has fifty-fifty been shown to notice pathology not seen on FA. In the future, faster scanning speeds would be crucial to obtain larger fields of view with higher resolution. More studies are needed to decide OCTA's utility in the clinical setting and to decide if this technology may offer a non-invasive option of visualizing the retinal vasculature in particular.
Abbreviations
- AMD:
-
Historic period-related macular degeneration
- BRAO:
-
Branch retinal artery occlusion
- BRVO:
-
Branch retinal vein occlusions
- CRAO:
-
Cardinal retinal artery apoplexy
- CRVO:
-
Cardinal retinal vein occlusions
- CSCR:
-
Central serous chorioretinopathy
- CNV:
-
Choroidal neovascularization
- FA:
-
Fluorescein angiography
- FAZ:
-
Foveal avascular zone
- GCL:
-
Ganglion jail cell layer
- GA:
-
Geographic atrophy
- ICGA:
-
Indocyanine green angiography
- INL:
-
Inner nuclear layer
- IPL:
-
Inner plexiform layer
- ILM:
-
Internal limiting membrane
- NVE:
-
Neovascularization elsewhere
- NVD:
-
Neovascularization of the disc
- NPDR:
-
Non-proliferative diabetic retinopathy
- OCT:
-
Optical coherence tomography
- OCTA:
-
Optical coherence tomography angiography
- OPL:
-
Outer plexiform layer
- RPED:
-
Retinal pigment epithelial disengagement
- PCV:
-
Polypoidal choroidal vasculopathy
- PDR:
-
Proliferative diabetic retinopathy
- RAP:
-
Retinal angiomatous proliferation
- RNFL:
-
Retinal nerve fiber layer
- RPE:
-
Retinal pigment epithelium
- SD-October:
-
Spectral domain optical coherence tomography
- SSADA:
-
Split-spectrum amplitude decorrelation angiography
- SS-OCT:
-
Swept source optical coherence tomography
- VCSEL:
-
Vertical cavity surface emitting laser
References
-
Kim DY, Fingler J, Zawadzki RJ, Park SS, Morse LS, Schwartz DM, et al. Optical Imaging of the chorioretinal vasculature in the living human center. Proc Natl Acad Sci. 2013;110:14354–9.
-
Choi Westward, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman 5, et al. Choriocapillaris and Choroidal Microvasculature Imaging with Ultrahigh Speed OCT Angiography. Plos Ane. 2013;8:e81499.
-
Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, et al. Stage-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121:180–vii.
-
Spaide RF, Klancnik JM, Cooney MJ. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2014; E1-6. doi:x.1001/jamaophthalmol.2014.3616.
-
Novotny 60 minutes, Alvis DL. A method of photographing fluorescence in circulating claret in the human retina. Circulation. 1961;24:82–six.
-
Novotny HR, Alvis D. A method of photographing fluorescence in circulating blood of the human being center. Tech Doc Rep SAMTDR USAF Sch Aerosp Med. 1960;sixty–82:ane–iv.
-
Kogure One thousand, Choromokos Eastward. Infrared absorption angiography. J Appl Physiol. 1969;26(one):154–seven.
-
Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12(iii):191–223.
-
Staurenghi G, Bottoni F, Giani A. Clinical Applications of Diagnostic Indocyanine Green Angiography. In: Ryan SJ, Sadda SR, Hinton DR, editors. Retina. London: Elsevier Saunders; 2013. p. 51–81.
-
Johnson RN, Fu Ad, McDonald HR, Jumper Yard, Ai E, Cunningham ET, et al. Fluorescein Angiography: Basic Principles and Interpretation. In: Ryan SJ, Sadda SR, Hinton DR, editors. Retina. London: Elsevier Saunders; 2013. p. 2–50.
-
Do DV, Gower EW, Cassard SD, Boyer D, Bressler NM, Bressler SB, et al. Detection of New-Onset Choroidal Neovascularization Using Optical Coherence Tomography: the AMD DOC Report. Ophthalmology. 2012;119:771–8.
-
Kotsolis AI, Killian FA, Ladas ID, Yannuzzi LA. Fluorescein Angiography and Optical Coherence Tomography Concordance for Choroidal Neovascularization in Multifocal Choroiditis. Br J Ophthalmol. 2010;94:1506–8.
-
Exercise DV. Detection of New-Onset Choroidal Neovascularization. Curr Opin Ophthalmol. 2013;24:224–vii.
-
Bressler NM, Bressler SB. Neovascular (Exudative or "Wet") Age-Related Macular Degeneration. In: Ryan SJ, Sadda SR, Hinton DR, editors. Retina. London: Elsevier Saunders; 2013. p. 1183–212.
-
Yuan A, Kaiser PK. Branch Vein Occlusion. In: Ryan SJ, editor. Retina. fifth ed. Mainland china: Saunders; 2013. p. 1029–38.
-
Kwiterovich KA, Maquire MG, Potato RP, Schachat AP, Bressler NM, Bressler SB, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991;98:1139–42.
-
Lopez-Saez MP, Ordoqui Eastward, Tornero P, Baeza A, Sainza T, Zubeldia JM, et al. Fluorescein-Induced Allergic Reaction. Ann Allergy Asthma Immunol. 1998;81:428–xxx.
-
Matsunaga D, Puliafito CA, Kashani AH. October Angiography in Good for you Human Subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(six):510–5.
-
Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, et al. Quantitative Optical Coherence Tomography Angiography of Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmology. 2014;121:1435–44.
-
Moult E, Choi West, Waheed NK, Adhi M, Lee B, Lu CD, et al. Ultrahigh-Speed Swept-Source Oct Angiography in Exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(half dozen):496–505.
-
de Carlo TE, Bonini Filho MA, Chin AT, Adhi K, Ferrara D, Baumal CR, et al. Spectral Domain Optical Coherence Tomography Angiography (OCTA) of Choroidal Neovascularization. Ophthalmology. In press.
-
Jia Y, Morrison JC, Tokayer J, Tran O, Lombardi Fifty, Baumann B, et al. Quantitative OCT Angiography of Optic Nerve Caput Blood Flow. Biomed Opt Express. 2012;3(12):3127–37.
-
Jia Y, Wei Eastward, Wang X, Zhang X, Morrison JC, Parikh Chiliad, et al. Optical Coherence Tomography Angiography of Optic Disc Perfusion in Glaucoma. Ophthalmology. 2014;seven(121):1322–32.
Acknowledgements
The authors gratefully acknowledge Dr. James G. Fujimoto, Eric Moult, Chen Lu, Jonathan Liu, Alex Cable, and Vijaysekhar Jayaraman for developing the prototype SS-OCTA system and processing the images used in iii of our figures. Additionally we gratefully acknowledge Dr. Caroline R. Baumal and Dr. Andre J. Witkin who each provided ane patient case included in this review.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
Fiscal Support: This work was supported in office past a Research to Prevent Blindness Unrestricted grant to the New England Middle Center/Section of Ophthalmology, Tufts University School of Medicine, and the Massachusetts Lions Clubs.
Financial Disclosure: Jay S. Duker is a consultant for and receives inquiry support from Carl Zeiss Meditech, Inc. and OptoVue, Inc.; André Romano is a consultant for and receives research support from OptoVue, Inc.
Authors' contributions
TED drafted the article and created the figures. AR, NKW, and JSD each contributed to the formulation and pattern of the article and critically revised the manuscript. All authors read and canonical the concluding manuscript.
Rights and permissions
Open Access This commodity is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, every bit long as y'all give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were fabricated.
The images or other 3rd party material in this commodity are included in the commodity's Creative Eatables licence, unless indicated otherwise in a credit line to the material. If material is not included in the commodity's Creative Commons licence and your intended utilise is not permitted by statutory regulation or exceeds the permitted apply, you will need to obtain permission straight from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
The Artistic Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the information made available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
Well-nigh this commodity
Cite this commodity
de Carlo, T.E., Romano, A., Waheed, N.K. et al. A review of optical coherence tomography angiography (OCTA). Int J Retin Vitr one, 5 (2015). https://doi.org/10.1186/s40942-015-0005-8
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s40942-015-0005-8
Keywords
- Age-related macular degeneration
- Diabetic retinopathy
- Fluorescein angiography
- Glaucoma
- Indocyanine angiography
- Optical coherence tomography angiography
- Optic disc
- Retina
- Retinal vessel occlusion
Source: https://journalretinavitreous.biomedcentral.com/articles/10.1186/s40942-015-0005-8
0 Response to "A Major Review of Optical Coherence Tomography Angiography"
Post a Comment